Parkinson’s Disease
Authors:
Christina Ledbetter, Shilka Babu, Hoang Nguyen, Richard Zweig, Owen Carmichael, Karen Sigvardt, Elizabeth Disbrow.
INTRODUCTION
There is growing evidence that disruptions in network connectivity may underlie cognitive deficits in Parkinson’s disease (PD). While default mode network (DMN) and central executive network (CEN) connectivity has been shown to be decreased in PD, less is known about the salience network (SN; Disbrow et al., 2014; Shine et al., 2011). The DMN, which includes the medial prefrontal and posterior cingulate cortex, is inactive during cognitively demanding tasks (Greicius et al., 2003;). On the other hand the CEN, which includes the dorsolateral prefrontal cortex and posterior parietal cortex, is active during tasks that require attention (Goulden et al., 2014; Menon and Uddin, 2010). The SN is hypothesized to play a role in switching between executive control and default mode networks. It includes two major cortical nodes, the anterior insula and the dorsal anterior cingulate cortex (dACC; Ham et al., 2013). The anterior insula is important in accessing memory and facilitating cognitive processes, while the dACC works to detect an error and make a behavioral adaptation in an instant (Christopher et al., 2013; Ham et al., 2013).
Using Granger causal analysis to study the effect of the SN cortical nodes on other brain regions, it was found that the right anterior insula of the SN plays an important role in switching between the activation of CEN and deactivation of DMN during cognitive processes (Menon and Uddin, 2010; Sridharan et al., 2008). To better understand the function of switching between the CEN and DMN by the SN, Goulden and colleagues (2014) conducted an analysis on two different subject populations: (1) 42 healthy participants from Bangor University and (2) 44 participants from the free NITRC database. They used independent component analysis and nonlinear dynamic causal modeling to measure connectivity and activity of the three brain networks: SN, DMN, and CEN. The neuroimaging analysis by Statistical Parametric Mapping (SPM8), GIFT toolbox, and Bayesian Model Selection of resting state scans, confirmed the model of SN causing the switch between the CEN and DMN compared to CEN or DMN being responsible for switching between brain networks.
Parkinson’s Disease (PD) was originally categorized as a motor disorder but now also includes cognitive deficits in memory, verbal, visuospatial processing, executive function, and processing speed (Collins and Williams-Gray, 2016; Disbrow et al., 2014). Nearly 25% of PD patients are diagnosed with mild cognitive impairment (MCI), which can be very detrimental to their quality of life and an indicator for PD dementia (PDD; Kalbe et al., 2016). One of the main cognitive deficits is attentional inflexibility, which is when PD patients are unable to switch attention from one domain to another (Fallon et al., 2016).
One of the brain networks involved in cognitive deficits like switching is the attentional network, which functions in spatial orienting, alerting, and executive control that can all be tested with the Attention Network Test (ANT; Cristinzio et al., 2013). In a study comparing 44 PD patients to 28 healthy control participants, the PD patients had slower reaction times in the orienting function of the attentional network (Zhou et al., 2012). Orienting problems, like being unable to identify a target at an uncued position, can be one of the first symptoms in degenerative disorders (Fernandez-Duque and Posner, 2001). In addition, attentional impairments such as lack of focus can be one of the first signs of PD that can be identified using neuropsychological tests and reaction time tasks, like the ANT where PD patients had slower response times that controls (Cristinzio et al., 2013).
PD is characterized by loss of dopamine in the striatum resulting in reduced availability of Dopamine 2 (D2) receptors specifically in the right anterior insula (Christopher et al., 2014). The anterior insula, which is part of the SN, is important to cognitive functions; therefore, decreased D2 receptors leads to executive dysfunction (Christopher et al., 2014). The depletion of D2 receptors in the right anterior insula is also directly related to the executive performance on neuropsychology tests in PD patients with mild cognitive impairment (MCI) showing the importance of the right anterior insula in cognitive processes (Christopher et al., 2014).
Increased activity in the anterior insula is associated with hyperactivity as seen during auditory verbal hallucinations in schizophrenic patients (Menon and Uddin, 2010; Sommer et al, 2008). Decreased activity in the anterior insula reduces its ability to combine multiple salient inputs and switch between CEN and DMN resulting in social dysfunction, which is associated with autism (Uddin and Menon, 2009). Therefore, unusual activity in the SN is related to disorders with impaired cognition.
Thus we propose to test the hypothesis that SN connectivity is disrupted in PD, and further, that connectivity is associated with performance of tasks requiring attention.
RESULTS
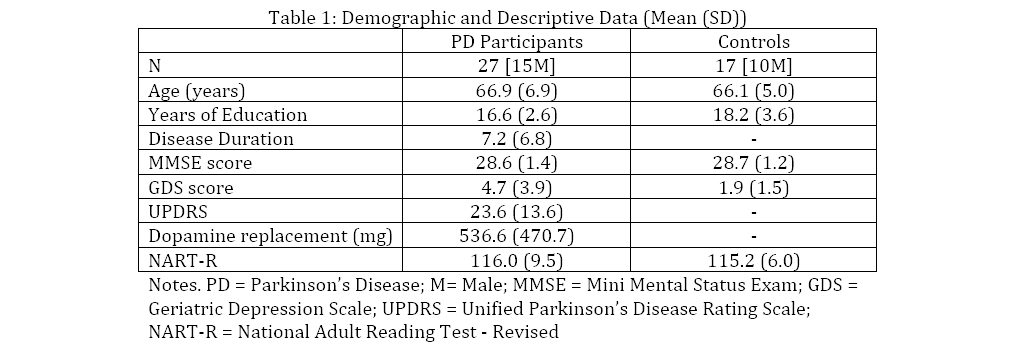
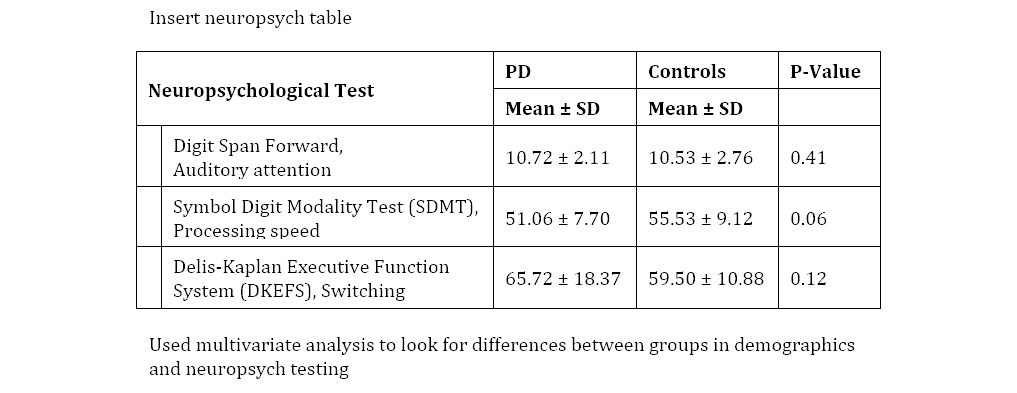
Behavioral Data
Group Differences in Functional Connectivity
In the PD group connectivity was significantly decreased between right dACC and both the left (z=-4.5) and right AI (z=-3.5). Similarly, connectivity was decreased between left dACC and the left (z=-3.8) and right AI (z=-3.9).
Connectivity and Neuropsychological Testing Performance
For auditory attention, in the control group greater task performance correlated with left dACC and left AI connectivity (z = 3.5. p<0.01). In the PD group greater task performance was correlated with greater left dACC and left (z=3.3) and right AI connectivity (z=4.3). A similar pattern was observed for the right dACC. Processing speed was negatively correlated with left dACC and left AI connectivity (z=-2.2) in controls. Right dACC connectivity was also correlated with left (z=3.3) and right (z=4.1) superior frontal gyrus BA9. In the PD group processing speed was negatively correlated with left dACC and right AI (z=-3.5) and left AI (z=-2.8) connectivity. The BA 9 connectivity correlation was absent in the PD group. Switching scores were positively correlated with both right and left dACC connectivity with left BA 10 (z=3.1; z=2.8) in controls. For the PD group, switching score were positively correlated with both right and left dACC connectivity with bilateral AI and bilateral area 10 (all z > 3).
[F(1, ##)=f-value, p=##]
DISCUSSION
Thus connectivity in the SN was decreased in PD compared to controls, and increased cognitive performance was associated with increased connectivity across cognitive domains. The precise connectivity pattern observed in controls was less specific in PD.